A student completed an online simulation investigating how emission spectra are produced. Which of the following best describes the process of producing emission spectra?
A. Electrons in the atom are positively charged causing a release of energy.
Incorrect. Electrons are negatively charged and do not release energy based solely upon their charge.
B. As electrons in the atom jump to a higher energy level they release energy.
Incorrect. Try to remember when the energy is released during the process.
C. As electrons in the atom drop to a lower energy level they release energy.
Correct! The electron releases the energy that brought it to the excited level.
D. Electrons in the atom are negatively charged causing a release of energy.
Incorrect. While electrons are negatively charge, the charge is not related to the release of energy.
Why is the study of emission spectra useful for identifying the elements that make up stars in outer space?
A. Each element emits photons with different proton levels.
Incorrect. Remember that photons in emission spectra are related to electrons.
B. Each element emits photons of different wavelength frequencies.
Correct! The emission spectrum of an element is like a fingerprint—no two are alike.
C. Each element emits photons of similar energy levels.
Incorrect. Remember that we see different wavelengths from different elements.
D. Each element contains a particular number of electron levels.
Incorrect. While this may be true in general, there is a missing part to this statement.
While looking through the tubes, they noticed that the diffraction grating produced a series of —
A. white lines known as refractors
Incorrect. Refractors deal with alteration of light paths.
B. white lines known as the visible line spectrum
Incorrect. The lines would be colored.
C. colored lines known as refractors
Incorrect. Refractors deal with alteration of light paths.
D. colored lines know as the visible line spectrum
Correct! This is the emission spectrum.
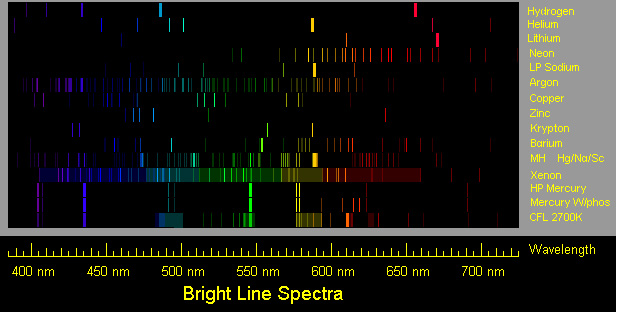
How do the emission spectra of Hydrogen and Neon compare in terms of wavelength?
A. Both Hydrogen and Neon have a high percentage of shorter wavelengths.
Incorrect. Look again at the image and the wavelengths in terms of nanometers.
B. Neon has a higher percentage of shorter wavelengths than Hydrogen.
Incorrect. Look again at the chart and the wavelengths in terms of nanometers.
C. Hydrogen has a higher percentage of shorter wavelengths than Neon.
Correct! There are more lines on the left side of the pattern for hydrogen.
D. Both Hydrogen and Neon have a high percentage of longer wavelengths.
Incorrect. Look again at the image and the wavelengths in terms of nanometers.

The emission spectrum for Xenon has many different emission lines. What can you infer about this property of Xenon?
A. It has a high density.
Incorrect. Think about how an emission spectrum is produced.
B. Its electrons have a high number of energy exchanges due to the number of energy levels.
Correct! More energy levels will correspond to more lines.
C. Its electrons have a low number of energy exchanges due to the number of energy levels.
Incorrect. Think about how an emission spectrum is produced.
D. It has a very large volume.
Incorrect. Think about how an emission spectrum is produced.